Why pH Matters in Cleaning: The Science, Simplified!
Every day, we encounter various cleaning challenges, from stubborn stains on our favorite surfaces to unexpected cleaning successes. We often attribute these outcomes to luck, brand quality, or friends’ recommendations. However, the real influencer behind these cleaning mysteries is a factor often overlooked: pH. This simple, two-letter term, perhaps a distant memory from high school […]

Every day, we encounter various cleaning challenges, from stubborn stains on our favorite surfaces to unexpected cleaning successes.
We often attribute these outcomes to luck, brand quality, or friends’ recommendations. However, the real influencer behind these cleaning mysteries is a factor often overlooked: pH. This simple, two-letter term, perhaps a distant memory from high school science classes, is the secret code for crucial information. It can make the difference between effectively cleaning and damaging your surfaces. A damaged surface looks dirty and becomes uncleanable, making the understanding of pH highly relevant to your cleaning success or failure.
Just as a key is designed for a specific lock, the suitability and effectiveness of a cleaning agent are significantly impacted by its pH level. pH measures the acidity or alkalinity of a liquid.
In this blog, we will dive into the fascinating world of pH and explore its significant Impact on Cleaning.
In this blog, we will delve into the fascinating world of pH and its significant impact on cleaning while also exploring how MARBLELIFE’s expertly formulated products and services offer a superior alternative to DIY methods like vinegar, baking soda, or lemon, providing the ideal solutions for your diverse cleaning needs.
Understanding the Impact of pH on Cleaning
Understanding that cleaners tackle dirt and oil in various ways is crucial. pH is a key factor, especially in the context of using acidity. While solvent-based cleaners aim to solubilize dirt and oils, they have become less popular due to efforts to reduce the use of VOCs (Volatile Organic Content). Consequently, pH-based cleaners are now favored over those relying on VOCs. Additionally, surfactant-based cleaners utilize a class of chemicals engineered so that one part of the molecule is attracted to water while the other is attracted to oil. This design allows the cleaner to surround and dissolve oil in water, making it easy to lift and remove from surfaces. However, the focus of this blog is specifically on pH-related cleaners.
You’ve likely used an acidic cleaner without realizing it when opting for vinegar, vinegar-infused cleaners, or citrus-scented products. Rust removers and hard water stain removers are also caustic. Masonry cleaners and grout haze removers can fall into this category as well. Why does this matter? Because we’re often in search of a do-it-all cleaner or experimenting to see if a new product can tackle a particular challenge. The catch is that using an acidic cleaner on a surface sensitive to acid can lead to costly or subtle damage. For instance, it might result in a hefty restoration bill for your marble countertop or the unintended removal of protective sealant from your granite counter or tile grout. Have you ever wondered why your grout is stained? It might be due to the one-time use of a low-pH cleaner.
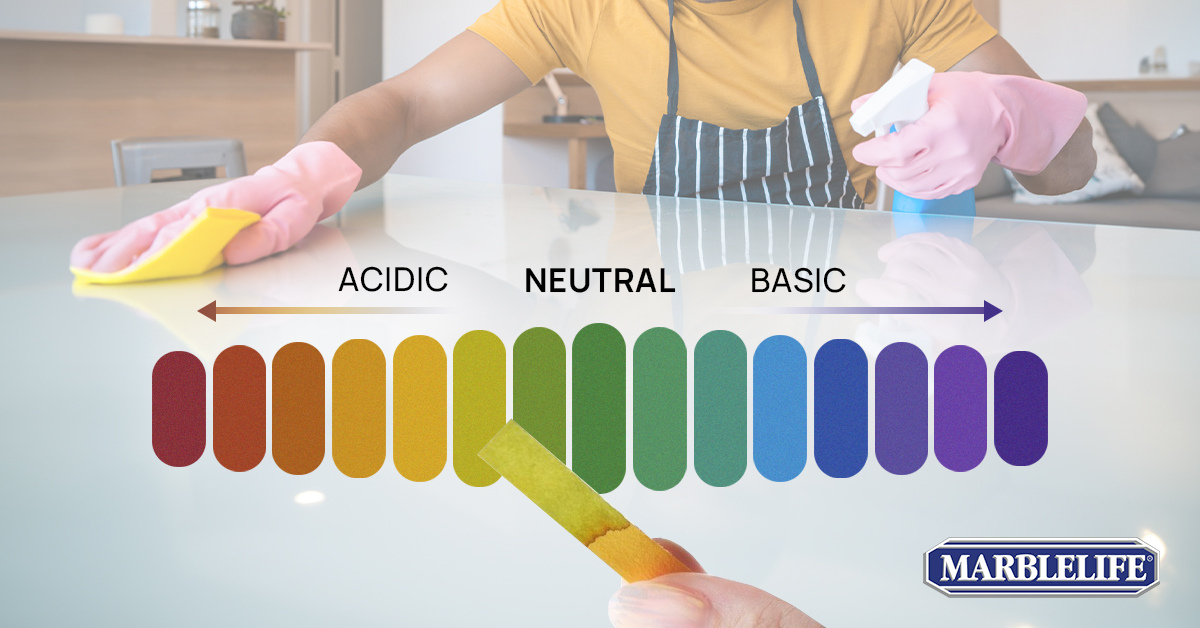
What is pH and Why Should You Care?
pH, “potential of hydrogen,” is a scale that measures a solution’s acidity or alkalinity (basicity), ranging from 0 to 14. At the midpoint of this scale is 7, representing neutrality, akin to distilled water. Substances with a pH lower than 7 are acidic (like lemon juice or vinegar), while those with a pH above 7 are basic or alkaline (such as many hand soaps). But why is this significant in your daily cleaning routine?
The effectiveness of a cleaning product in removing stains, combating bacteria, and safely interacting with various surfaces is heavily influenced by its pH level. Our skin, slightly acidic with a pH of around 5.5, benefits from products that align with its natural balance. This balance ensures effective cleaning and safeguards our skin’s health and the integrity of the surfaces we clean. Understanding pH helps us become better cleaners and more informed and health-conscious consumers.
In this context, MARBLELIFE’s products stand out. They are scientifically formulated to achieve the perfect pH balance for different surfaces. This approach ensures both the safety and effectiveness of the cleaning process, making these products a superior choice for discerning users.
Understanding the pH Scale
The pH scale is essentially a spectrum. Imagine it as a sliding scale with distilled water at its center, marking the neutral point with a pH of approximately 7.
As we move lower on the scale, substances become increasingly acidic, such as the tangy lemon juice with its sour bite or the vinegar we use in our salad dressing.
Ascending the scale takes us to basic or alkaline substances, like the gentle soap lather. Grasping this scale is like possessing a decoder ring for the cleaning world, enabling you better to match the right cleaning agent to the specific task.
MARBLELIFE’s product range is expertly crafted to fit within the ideal pH range for each surface, from marble to vinyl, ensuring effective cleaning without risking damage.
The Intricacy of pH in Cleaning
Envision the cleaning process as a delicate balancing act, akin to a seesaw in a playground. On one side, imagine acidic residues like wine spills or fruit stains, each with unique characteristics. On the opposite end, envision basic grime, such as soap scum or specific food residues. To achieve a balanced, effective cleaning, you need a cleaning agent that acts as the perfect counterweight to the nature of the stain or dirt.
A more alkaline cleaner often does the trick for an acidic spill, and vice versa. This cleaning process isn’t just moving dirt around; it’s about neutralizing its acidity or bringing it to a less active pH of 7 and lifting it from the surface. This precise art of leveraging pH transforms ordinary cleaning into a scientific triumph, ensuring spaces are not just tidy but truly clean.
If you remember your high school chemistry, the acid reaction with a base generates a salt closer to neutral on the scale. Another way to manage pH was developed by MARBLELIFE’s predecessor, Union Carbide, who developed a surfactant-based means of hiding acid to neutralize or block its effect. As noted above, a surfactant can surround a chemical and prevent it from interacting with water or its surroundings. Once surrounded, it is neutralized from acting, even though it may still exist as an acid within the surrounding surfactant. This process proved to be a game-changing improvement in technology capable of neutralizing acidic spills and lifting and solubilizing oil from a surface with the same technology. Non-acidic, solubilizing technology translated to faster, more effective cleaners. MARBLELIFE pioneered the technology used today in MARBLELIFE’s cleaners and is seen in various other cleaners in other industries across the country.
That said, with the best cleaning technology on the planet, Union Carbide would then learn a critical lesson that would result in the need to develop and launch marble restoration and hard surface restoration processes, and ultimately, MARBLELIFE was found. The lesson? Damaged surfaces look dirty.
Risks of Ignoring pH in Cleaning
Cleaning without proper knowledge or consideration of pH is akin to navigating a tightrope while blindfolded. You may be making progress, but the potential for missteps is significant. Using a cleaning product with an inappropriate pH level can do more than fail to clean effectively—it can actively harm your surfaces, potentially leading to thousands of dollars in damage. This risk includes increased chances of encountering stained grout or stains on granite countertops.
Consider marble, a surface particularly vulnerable to acid. Applying an acidic cleaner on marble can lead to etching or discoloration, significantly diminishing its pristine beauty. This vulnerability is also for limestone, travertine, concrete, and cementitious terrazzo. Damage can manifest as water spots, rings, spills, or splatter marks. Light etches might feel smooth, but deeper ones can feel rough. Factors such as the acid’s concentration, the duration it remains on the surface before being cleaned, or the frequency of contact can influence the extent of damage (it’s advisable not to clean with vinegar).
Similarly, certain metals or finishes may tarnish or corrode when exposed to overly acidic or alkaline solutions. Imagine a once-gleaming countertop now dulled or a shiny floor becoming patchy—all due to an incorrect pH choice. Understanding and respecting the role of pH in cleaning is not just important; it’s crucial to maintaining the integrity and appearance of various surfaces.
Marble, Granite, and Their pH Sensitivity
Marble, primarily composed of calcium carbonate, is susceptible to acids. Even a minor spill of lemon juice, if not promptly addressed, can etch its surface, replacing its characteristic shine with a dull finish. Granite, while more resilient, is not impervious to the adverse effects of improper pH levels, which can lead to surface dullness or degradation.
Consider the example of the Cheesecake Factory, which once had tan marble tables. Serving water with lemon wedges, they noticed the tables appeared dirty over time. The acidic lemon water created dull spots and splatter marks, eventually rendering these expensive tables dull and unattractive. Today, these tables have either been coated or replaced.
Granite, though less sensitive to acids, has its vulnerabilities. The penetrating sealer used for granite protection can be eroded by acidic substances, exposing the stone to potential staining in the future.
Acknowledging the delicate nature of these materials, MARBLELIFE has developed products specifically designed for their care. The MARBLELIFE® Marble & Travertine InterCare Cleaner is an excellent choice for marble. It uses surfactant-based cleaning technology to offer a non-acidic, stone-safe cleaner that removes oil and dirt and neutralizes acidic spills. Easy to use, effective, streak-free, wax-free (to prevent build-up), and oil-free, this cleaner’s “InterCare” based technology ensures comprehensive cleaning of natural stone surfaces without the risk of pH-induced damage typical of acidic cleaners. It leaves a beautiful, streak-free shine and is safe for the user and the stone surface.
For granite surfaces, the MARBLELIFE® Granite Countertop Cleaner is particularly effective. It is specially formulated to remove oils, grease, food, and adhesives from the tiny pores of kitchen counters or bathroom vanities. This unique formulation ensures that granite and quartz surfaces maintain their beauty through effective cleaning without risking sealer protection or experiencing wax build-up over time, a common issue with glass cleaners.
MARBLELIFE’s pH-Balanced Solutions
MARBLELIFE distinguishes itself with its specialized, pH-balanced solutions. With decades of expertise in surface care, MARBLELIFE has developed a range of products that effectively clean while preserving the integrity of various surfaces. Whether it’s marble, granite, or other materials, each MARBLELIFE product results from meticulous research and development, specifically formulated to address the unique pH sensitivities of each type of material.
This commitment goes beyond just maintaining surface beauty. MARBLELIFE is devoted to ensuring the health and safety of the environments where these surfaces are used. The company provides household-friendly products, steering clear of harsh chemicals. This approach reflects MARBLELIFE’s dedication to the aesthetic appeal of surfaces and the well-being of the users and their surroundings.
DIY Cleaners: The pH Factor in Cleaning
The appeal of DIY cleaning solutions like vinegar and baking soda is undeniable. Embracing green cleaning methods is commendable, but ensuring that these methods are both non-damaging and effective is crucial. While these household items have their uses, they are unsuitable for surfaces like marble, travertine, limestone, or porous surfaces requiring a penetrating sealer, such as granite and grout.
With its acidic nature (pH around 2-3), vinegar might be effective as a glass cleaner but can damage sensitive surfaces like marble or limestone. On the other hand, baking soda, being basic (pH around 9), can effectively tackle grease but might leave chalky residues or gradually abrade surfaces over time. Therefore, while these DIY solutions have their merits, it’s vital to understand their pH implications and limitations.
In contrast, MARBLELIFE’s products offer a scientifically sound and safer alternative. These products are specifically designed to avoid the risks associated with extreme pH levels. By choosing MARBLELIFE’s tailored solutions, you can clean confidently, knowing that your surfaces are being treated with products that maintain their integrity and beauty without the adverse effects of unsuitable pH levels.
Conclusion
Grasping the concept of the pH scale goes beyond mere scientific knowledge; it’s a crucial aspect of enhancing your cleaning routine. Steering clear of acidic cleaners (those with a pH less than 7) is more important than just seeking ‘neutral’ cleaners. Avoiding low-pH solutions is vital for preserving the beauty, integrity, and longevity of various surfaces, whether in your home or a commercial setting.
This situation is where MARBLELIFE’s expertise truly stands out. Our non-acidic cleaning products represent more than just cleaning solutions; they embody our commitment to science, care, and preserving your valuable surfaces. We encourage you to experience the difference MARBLELIFE can bring to your cleaning regime. Discover our scientifically formulated solutions at marblelifeproducts.com, where science and the art of cleaning converge.
Unsure about your surface, cleaner, or situation?
If you’re uncertain whether your space requires a specialized MARBLELIFE product or the expertise of our technicians? Distinguishing between surface damage and mere dirt can sometimes be a challenge. In such instances, MARBLELIFE is ready to assist with a FREE CONSULTATION to help you make the best decision for your space. Our knowledgeable team will guide you in selecting the appropriate product or service, ensuring that your surfaces are visually pristine and treated with the highest level of care.
Don’t let uncertainty hold you back or risk expensive damage. Experience the pinnacle of surface care, which means enjoying a worry-free environment. Visit our website to schedule your free consultation today. With MARBLELIFE, you’re not just cleaning but nurturing a legacy of brilliance and preservation for your surfaces.












